Introduction:
Germline DEAD-box RNA helicase-1 mutant (DDX41) predisposition syndrome ( DDX41-MT-GPS) is one of the most frequent hereditary predisposition syndromes in adults with myeloid neoplasms (MN-2-5%). Somatic mosaicism involving the unmutated DDX41 allele (e.g., R525H) as a potential mechanism for myeloid evolution has been reported in 39-50% of patients (pts). In addition, given the later age of onset, somatic mosaic states composed of age-related clonal hematopoiesis (ARCH) (e.g., DNMT3A, ASXL1, TET2, and TP53), with reported frequencies less than expected in MDS and AML, have been documented. We conducted this study to define the somatic mosaic landscape in DDX41-MT-GPS .
Methods:
After approval from the Mayo Clinic IRB the prospective GPS database was queried for pts with DDX41-MT GPS. Germline confirmation was conducted on skin fibroblast-, or hair follicle-derived DNA. Somatic CH testing was conducted using a targeted NGS panel as previously described. Given the high frequency of variants of undetermined significance (VUS), comparisons were made between pathogenic ( DDX41path) and DDX41VUS.
Results: We identified 106 pts with DDX41-MT-GPS, of which 83 (78%) met criteria for MN, 16 (15%) for CH/CCUS (clonal cytopenias of undetermined significance), while 7 (6%) were unaffected carriers.
Somatic mosaicism in DDX41-GPS patients without MN
Sixteen DDX41-MT pts without MN met criteria for CH/CCUS ( DDX41path [n=4] and DDX41VUS [n=12]; median age 69 years (yrs) (range [R], 19-78) ( DDX41path [69 yrs] and DDX41VUS [67 yrs], p=0.85), with a male predilection (75%). Four (25%) pts had somatic DDX41 MT (R525H [n=3] and A376T [n=1]) with median variant allele frequency (VAF) 10.5% (R, 5-27); 2 (50%) pts with germline DDX41path had R525H (VAF; 5% and 13%) and 1 (8%) pt each with germline DDX41VUS had R525H (VAF 5%) and A376T (VAF 27%) somatic variants, respectively. Abnormal cytogenetics (CG) were observed in 2/13 evaluable (15%) pts ([+8 and del20q]) in the germline DDX41path group only (p= 0.05). ARCH co-mutations were observed in 11 (69%) pts, not significantly (NS) different between DDX41path (75%) and DDX41VUS (75%), p= 0.66. Individual co-mutations: DNMT3A (25% vs 16%, p= > 0.99), ASXL1 (25% vs 8%, p=0.71), TET2 (25% vs 8%, p= >0.99), and JAK2 (0% vs 8%, p= >0.99) were NS different between the two groups.
Somatic mosaicism in DDX41-GPS patients with MN
Eight-three pts met criteria for MN ( DDX41path [n=43] and DDX41VUS [n=40]; median age 67 yrs (range [R], 27-92), with a male predilection (58%). Fifty-four (65%) pts had MDS ( DDX41path [55%] and DDX41VUS [45%], p=0.55) and 29 (35%) had AML ( DDX41path [41%] and DDX41VUS [59%], p=0.65). Overall, 15 (18%) pts had somatic DDX41 MT; 12 (80%) pts with DDX41path and 3 (20%) pts with DDX41VUS (p= 0.003). The median VAF of somatic DDX41 MT was 12% (R, 5-37), NS different between DDX41path (10.5%) and DDX41VUS (12%) (p=0.08). The most common somatic DDX41 MT was R525H (66% [n=10]), significantly associated with DDX41path (9/10 [90%]), p=0.01. ARCH was observed in 17 (20%) pts, significantly higher in DDX41path (76%) compared to DDX41VUS (24%), p= 0.03. Abnormal CG were observed in 10/73 evaluable (14%) pts (-Y [n=2], del 20 [n=3], del 5q [n=2], del 7q [n=1], t (3;8) [n=1] and complex CG [n=1]), higher in DDX41path (70%) compared to DDX41VUS (30%) with p value >0.99.
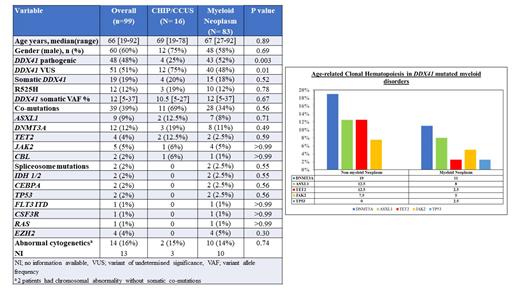
We compared the spectrum of CH between DDX41 MT CH/CCUS and MN pts ( Table 1) and found no difference in the proportion of pts with somatic DDX41 MT (p= 0.52) and ARCH/myeloid MT (p= 0.56). The individual occurrences of DNMT3A (p= 0.49), ASXL1 (p= 0.71), TET2 (p=0.59), JAK2 (p>0.99) and TP53 (p= 0.56) mutations were NS different between the two groups ( Figure 1).
Conclusion:
We define the somatic mosaic landscape in DDX41-MT GPS and demonstrate that somatic mutations involving the other allele, especially R525H, are seen in ~20% of pts (>80% of R525H present in MN). The frequency and composition of ARCH and myeloid driver mutations was lower than expected for the age of presentation, in comparison to de novo and secondary MDS/AML and occurred more frequently in DDX41path compared to DDX41VUS. Forty-two % (n=41), including 37% with MN, did not have somatic mosaicism, underscoring the fact that mechanisms of leukemia progression remain to be defined in subsets of affected pts.
Disclosures
Foran:Roivant: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Actinium: Research Funding; Kura: Research Funding; Sellas: Research Funding; Novartis: Research Funding; Celgene: Research Funding; Astellas: Research Funding; NCI: Membership on an entity's Board of Directors or advisory committees; CTI: Membership on an entity's Board of Directors or advisory committees. Alkhateeb:Mayo Clinic: Current Employment. He:Kura Oncology, Inc: Consultancy. Patnaik:StemLine: Research Funding; Epigenetix: Research Funding; Kura: Research Funding; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal